Why is titanium dioxide used in food, including in Skittles?
Over the last several years, nanoparticles have come under scrutiny for adverse health effects. Nanoparticles are ultrafine particles between 1 to 100 nanometers in diameter. (To put this in perspective, the average human hair is around 80,000 nanometers thick.) Because of their size, which can be engineered and manipulated at the atomic or molecular level, nanoparticles exhibit unique physical, chemical, and biological properties. Titanium dioxide is one of the most commonly produced nanoparticles in the world.
The CaCO3 and TiO2 factory not only provides a reliable supply of these materials to industries but also contributes to the local economy by creating job opportunities and generating revenue. The factory employs skilled workers in various departments such as production, quality control, and maintenance. It also collaborates with suppliers and distributors to ensure efficient transportation and delivery of CaCO3 and TiO2 to customers worldwide.
The US and Canada, however, approve the use of titanium dioxide as a food additive. Canada's recent review of titanium dioxide reconfirmed its safety and pointed out that many of the toxicity studies the EU reviewed were not relevant to the safety of titanium dioxide as a food ingredient, and that the ban is based on an abundance of caution and uncertainty.
Titanium dioxide in food is used in a variety of products as a color enhancer. The most common foods containing titanium dioxide include:
For the Fourth Quarter of 2021
Infrared analysis showed that the characteristics bands for the bare nanoparticles are still exhibited in the vitamins@P25TiO2NPs spectra, such as a wide peak in 450–1028 cm−1 related to the stretching vibration of Ti-O-Ti and other peaks in 1630 cm−1 and 3400 cm−1, which represent the surface OH groups stretching. The IR spectrum of vitaminB2@P25TiO2NPs showed signs of binding between compounds. The OH bending peak (1634 cm−1) corresponding to bare nanoparticles disappeared, and the NH2 bending band characteristic of vitamin B2 appeared (1650 cm−1). The IR spectrum of vitaminC@P25TiO2NPs also showed signs of successful functionalization. Bands at 1075 cm−1; 1120 cm−1; 1141 cm−1 were observed, which are originated by C O-C vibrations present in the vitamin C. The intense band at 1672 cm−1 is attributed to the C = O stretching in the lactone ring while the peak at 1026 cm−1 is ascribed to the stretching vibration Ti-O-C. Wide bands at 3880–3600 cm−1 are related to stretching vibration OH groups, but those disappear in the modified nanoparticles spectrum. These observations confirm the interactions between the P25TiO2NPs and the vitamins [35].
O-C vibrations present in the vitamin C. The intense band at 1672 cm−1 is attributed to the C = O stretching in the lactone ring while the peak at 1026 cm−1 is ascribed to the stretching vibration Ti-O-C. Wide bands at 3880–3600 cm−1 are related to stretching vibration OH groups, but those disappear in the modified nanoparticles spectrum. These observations confirm the interactions between the P25TiO2NPs and the vitamins [35].
Titanium dioxide is a naturally-occurring mineral found in the earth’s crust. Because of its white color, opaqueness, and ability to refract light, the ingredient is often used as a pigment, brightener, and opacifier, which is an ingredient that makes a formulation more opaque. Titanium dioxide is also a UV filter and so is an effective active ingredient in sunscreens. It’s often used in cosmetic loose and pressed powders, especially “mineral powder” cosmetics, in addition to other cosmetics, lotions, toothpaste, and soap.
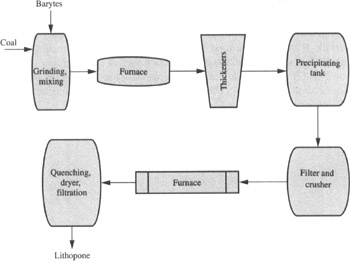
 lithopone raw material suppliers. We adhere to strict environmental regulations and take measures to minimize waste and emissions during the production process. By doing so, we not only protect the environment but also ensure the long-term sustainability of our business.
lithopone raw material suppliers. We adhere to strict environmental regulations and take measures to minimize waste and emissions during the production process. By doing so, we not only protect the environment but also ensure the long-term sustainability of our business. This ensures that the TiO2 pigment produced by these factories is of the highest quality and suitable for various applications This ensures that the TiO2 pigment produced by these factories is of the highest quality and suitable for various applications
This ensures that the TiO2 pigment produced by these factories is of the highest quality and suitable for various applications This ensures that the TiO2 pigment produced by these factories is of the highest quality and suitable for various applications ti02 pigment factories.
ti02 pigment factories.Applications
The aim of this work was to examine particularly the Degussa P25 titanium dioxide nanoparticles (P25TiO2NPs) because they are among the most employed ones in cosmetics. In fact, all kinds of titanium dioxide nanoparticles (TiO2NPs) have gained widespread commercialization over recent decades. This white pigment (TiO2NPs) is used in a broad range of applications, including food, personal care products (toothpaste, lotions, sunscreens, face creams), drugs, plastics, ceramics, and paints. The original source is abundant in Earth as a chemically inert amphoteric oxide, which is thermally stable, corrosion-resistant, and water-insoluble. This oxide is found in three different forms: rutile (the most stable and substantial form), brookite (rhombohedral), and anatase (tetragonal as rutile), of these, both rutile and anatase are of significant commercial importance in a wide range of applications [3]. Additionally, the nano-sized oxide exhibits interesting physical properties, one of them is the ability to act as semiconducting material under UV exposure. In fact, TiO2NPs are the most well-known and useful photocatalytic material, because of their relatively low price and photo-stability [4]. Although, this photoactivity could also cause undesired molecular damage in biological tissues and needs to be urgently assessed, due to their worldwide use. However, not all nanosized titanium dioxide have the same behavior. In 2007, Rampaul A and Parkin I questioned: “whether the anatase/rutile crystal form of titanium dioxide with an organosilane or dimethicone coat, a common titania type identified in sunscreens, is appropriate to use in sunscreen lotions” [5]. They also suggested that with further study, other types of functionalized titanium dioxide could potentially be safer alternatives. Later, Damiani found that the anatase form of TiO2NPs was the more photoactive one, and stated that it should be avoided for sunscreen formulations, in agreement with Barker and Branch (2008) [6,7].
 Advanced techniques such as hydrothermal synthesis, sol-gel processes, and chemical vapor deposition are employed to achieve the desired nanoscale dimensions and crystalline forms Advanced techniques such as hydrothermal synthesis, sol-gel processes, and chemical vapor deposition are employed to achieve the desired nanoscale dimensions and crystalline forms
Advanced techniques such as hydrothermal synthesis, sol-gel processes, and chemical vapor deposition are employed to achieve the desired nanoscale dimensions and crystalline forms Advanced techniques such as hydrothermal synthesis, sol-gel processes, and chemical vapor deposition are employed to achieve the desired nanoscale dimensions and crystalline forms anatase and rutile nano-tio2 factory.
anatase and rutile nano-tio2 factory.We've used titanium dioxide safely for decades. However, recently its safety was called into question.
At CRIS, we've explored the safety of titanium dioxide for nearly half a decade, including conducting double-blind research to test the safety of food-grade titanium dioxide (E171). Our study shows that when exposed to food-grade titanium dioxide in normal conditions, research animals did not experience adverse health outcomes.
It's important to emphasize that in a National Institutes of Health study, experimental animals were exposed to titanium dioxide in amounts as high as 5% of their diet for a lifetime and showed no evidence of adverse effects.
A handful of studies greatly influenced the decisions made by the European Food Safety Authority (EFSA). Unfortunately, these studies did not consider that titanium dioxide exposure comes from food, not drinking water. Additionally, CRIS researchers could not reproduce the adverse outcomes identified by the studies through typical food ingestion. Regardless, the EFSA banned E171 as a food ingredient and for use in other capacities in the summer of 2022.
In 2022, the United States, United Kingdom, and Canada maintained that the scientific evidence supports that titanium dioxide (E171) is safe for humans to use and consume.
An inorganic chemical, titanium dioxide is used as a dye to help products achieve a certain appearance, including whitening a product. Some experts and publications have described it as being akin to a paint primer that's used before the color is added to food in order to give products a uniform shine. Its presence is common in many items beyond Skittles including coffee creamers, cake mixes, and chewing gum. It's also used for pigment and in cosmetics manufacturing.